Metformin and Longevity: 8 Powerful Insights Backed by Science

The FDA approved metformin in 1994 to control blood sugar levels. Scientists found something amazing beyond its diabetes benefits. Research shows metformin might lower cancer risk, protect against dementia, and prevent strokes while slowing down aging. Female mice that started taking metformin early lived 8% longer on average, with their maximum lifespan increasing by 9%. People with diabetes who took metformin had better survival rates than those who didn’t. These results got researchers excited about metformin’s life extension possibilities. Now, the groundbreaking Targeting Aging with Metformin (TAME) Trial plans to study over 3,000 people aged 65 to 79.
Let’s get into the science behind Metformin and Longevity and look at evidence from studies that show why this affordable medication could be one of the most promising anti-aging compounds available. We’ll explore how it works – from making your body more sensitive to insulin to reducing inflammation – and what you should know about taking metformin to live longer.
Metformin’s Origins and Its Role in Diabetes Management

Image Source: Owlcation
Metformin’s story starts in medieval Europe’s herbal medicine cabinets, not in a modern lab. This simple medication, now central to metformin and longevity research, comes from a small flowering plant with a rich history.
Discovery from Galega officinalis and guanidine derivatives
Galega officinalis, also called French lilac or goat’s rue, was a common remedy in Europe for centuries. Medieval healers used this plant for many health issues, especially what we now know as diabetes symptoms. People called it “professor weed” because it helped reduce frequent urination and thirst—typical signs of diabetes.
Galega officinalis presents an interesting contrast—it helps humans but can kill livestock, especially goats (that’s why it’s called “goat’s rue”). The plant contains guanidines, compounds that lower blood sugar but are too dangerous for medical use.
Scientists made a breakthrough in the 1920s. They isolated the active ingredients in Galega and found their blood sugar-lowering properties. In 1922, they extracted guanidine from the plant, which showed it could lower blood sugar. In spite of that, guanidine proved too toxic for patients, so scientists created safer versions.
Their work led them to create biguanides—compounds with two linked guanidine groups—in the late 1920s. Three potential diabetes treatments emerged: phenformin, buformin, and metformin. Metformin (dimethylbiguanide) showed the best mix of effectiveness and safety.
FDA approval timeline and global adoption
Scientists created metformin in 1922, but it took decades to gain medical acceptance. French doctor Jean Sterne ran the first clinical trials in 1957 and named it “Glucophage” (glucose eater). France became the first country to approve metformin for diabetes treatment in 1958.
The drug faced big challenges on its way to global use. Phenformin and buformin were pulled from markets worldwide in the 1970s because they caused lactic acidosis—a potentially deadly side effect. This made people skeptical about all biguanides, which delayed metformin’s adoption even though it was much safer.
The FDA finally approved metformin in 1994 after reviewing years of European safety data. The 40-year gap between its creation and US approval stands out as one of medicine’s unusual timelines.
Metformin quickly became the go-to treatment for type 2 diabetes after FDA approval. The World Health Organization added it to their List of Essential Medicines. Today, it’s one of the most prescribed drugs globally, with over 120 million users.
Key factors in metformin’s success include:
- Generic versions cost less than $10 per month
- Lower risk of low blood sugar compared to other diabetes drugs
- No weight gain or slight weight loss
- Decades of safety data
- Heart health benefits
Current clinical uses beyond diabetes
Metformin helps with more than just diabetes. Scientists and doctors have found many other uses that connect with its anti-aging potential:
Polycystic Ovary Syndrome (PCOS): Metformin helps women with PCOS by improving insulin response and lowering androgen levels. This often helps restore ovulation and boosts fertility. Many doctors now use it as their first choice for PCOS treatment.
Cancer Prevention and Treatment: Studies show that people who take metformin get cancer less often. Lab tests suggest it stops cancer cells from growing through AMPK activation and mTOR inhibition—the same ways it might help with aging. Many clinical trials now look at metformin as an extra cancer treatment.
Cardiovascular Protection: Metformin helps heart health beyond just lowering blood sugar. The UK Prospective Diabetes Study found it cut heart attack risk by 39% compared to other treatments that controlled blood sugar just as well.
Weight Management: Unlike other diabetes drugs that cause weight gain, metformin often helps people lose some weight. This makes it useful for people with metabolic syndrome who aren’t diabetic but need help with weight.
Neurodegenerative Disease Prevention: New research hints that metformin might lower dementia risk and other brain diseases, possibly by reducing inflammation and helping cells use energy better.
Anti-Aging Research: The Targeting Aging with Metformin (TAME) trial marks the first FDA-approved study to test if a drug can delay age-related diseases. This excites many people interested in metformin for longevity.
These uses share common ground in how metformin affects cell energy and inflammation—key processes in both disease and aging.
Additional Resources:
- American Diabetes Association – Metformin Guidelines
- National Institute on Aging – TAME Trial Information
- FDA Prescribing Information for Metformin
AMPK Activation and Cellular Energy Regulation
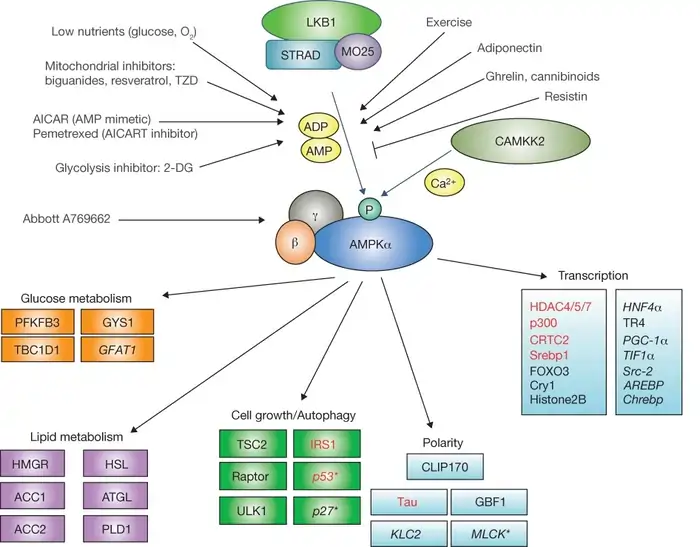
Image Source: Nature
Metformin releases its potent effects at the cellular level through a fascinating mechanism. The process centers on an enzyme called AMP-activated protein kinase (AMPK)—the cell’s main energy sensor and a crucial player in metformin’s potential longevity benefits.
AMP/ATP ratio and AMPK stimulation
AMPK works as a cellular “fuel gage” that monitors energy levels constantly. This enzyme exists as a heterotrimeric complex with one catalytic α-subunit and two regulatory β- and γ-subunits [1]. The enzyme activates to restore balance when cellular energy runs low, which AMP/ATP or ADP/ATP ratios indicate.
Metformin activates AMPK by inhibiting complex I of the mitochondrial electron transport chain [1]. This inhibition reduces ATP production and increases cellular AMP/ATP and ADP/ATP ratios. Rising AMP levels bind to AMPK’s γ-subunit and cause a critical change in shape that:
- Promotes phosphorylation at Thr172 of the α-subunit by upstream kinases
- Prevents dephosphorylation by protein phosphatases
- Enables allosteric activation by AMP binding
This three-pronged mechanism creates extreme sensitivity to even small changes in the cell’s energy status [2]. Metformin can activate AMPK at both high and clinical doses through different mechanisms. High concentrations (≥250 mg/kg in mice) cause substantial energy depletion with clear changes in AMP/ATP ratios [1]. Clinical doses (5-30 μM in plasma) work through a lysosomal mechanism with vacuolar H+-ATPase inhibition [1]. This explains its effectiveness at concentrations that don’t substantially alter overall energy ratios.
LKB1–AMPK–CRTC2 pathway in glucose metabolism
AMPK phosphorylates many downstream targets to regulate glucose metabolism after activation. The liver kinase B1 (LKB1)–AMPK–CRTC2 pathway is central to metformin’s anti-diabetic and potential anti-aging effects.
LKB1 is the main upstream kinase that activates AMPK when energy depletes [3]. AMPK gets activated by LKB1 and phosphorylates CRTC2 (CREB-regulated transcription coactivator 2). This makes CRTC2 move from the nucleus to the cytoplasm [4]. The process disrupts CRTC2’s ability to coactivate CREB-dependent transcription of gluconeogenic genes.
This pathway shows how metformin reduces hepatic glucose production—a vital function for diabetes management and potentially longevity. Activated AMPK acts like a metabolic switch that turns off energy-consuming processes like gluconeogenesis when energy is scarce [5].
AMPK’s role in lipid oxidation and insulin sensitivity
AMPK plays a crucial role in lipid metabolism and insulin sensitivity beyond glucose regulation. These factors closely link to metformin’s anti-aging potential. Activated AMPK stops fat synthesis and encourages fat oxidation by directly phosphorylating acetyl-CoA carboxylase (ACC) [6].
ACC comes in two isoforms: ACC1 controls fatty acid synthesis, while ACC2 regulates fatty acid oxidation. AMPK phosphorylates both at inhibitory sites (Ser79 on ACC1 and Ser221 on ACC2) [7]. This dual action:
- Decreases malonyl-CoA production and removes CPT1 inhibition
- Allows more fatty acids to enter mitochondria for oxidation
- Reduces lipogenesis and liver fat buildup
Scientists showed this mechanism’s importance for metformin’s effects in knock-in mice with non-phosphorylatable ACC. These mice developed insulin resistance and stopped responding to metformin’s metabolic benefits. Normal mice showed substantial improvements in insulin sensitivity after metformin treatment [6].
AMPK activation by metformin boosts glucose uptake in muscles through increased GLUT4 movement to the plasma membrane [8]. This insulin-independent pathway offers another way to improve glucose balance.
These molecular mechanisms explain why metformin improves metabolic parameters and might extend lifespan. It mimics aspects of caloric restriction—a proven intervention that extends lifespan in multiple species.
Additional Resources:
- American Diabetes Association – Scientific Sessions (AMPK Research)
- National Institute on Aging – Targeting Aging with Metformin Trial
- Cell Metabolism Journal – AMPK Signaling Research
mTORC1 Inhibition and Protein Synthesis Control
Our cells’ protein-building machinery contains crucial clues about how metformin might help us live longer. The mammalian target of rapamycin complex 1 (mTORC1) plays a central role here. This master regulator of cell growth gets efficiently shut down by metformin.
AMPK-dependent inhibition of mTORC1
Metformin’s activation of AMPK sets off a chain of events that shuts down mTORC1. This happens through two main pathways:
The first pathway starts when activated AMPK phosphorylates tuberous sclerosis complex 2 (TSC2) at Ser-1387. This enhances its GTPase-activating protein (GAP) activity toward Rheb – a key mTORC1 activator [9]. The phosphorylation changes Rheb from its active GTP-bound state to an inactive GDP-bound form, which stops it from activating mTORC1.
The second pathway involves AMPK directly phosphorylating Raptor (a core mTORC1 component) at Ser-792. This makes it bind with inhibitory 14-3-3 proteins [10]. The binding shuts down mTORC1 kinase activity without involving the TSC complex.
Lower metformin doses (100 mg/kg) need the TSC complex to inhibit mTORC1. Scientists discovered this in TSC1-knockout mice where metformin couldn’t suppress mTORC1 signaling [11]. Higher doses can still partly inhibit mTORC1 through direct Raptor phosphorylation, even without TSC2. This dose relationship matters a lot for people taking metformin to live longer.
REDD1 and Rag GTPase pathways
Metformin doesn’t just work through AMPK. It has other ways to block mTORC1 that could help slow aging:
The Regulated in DNA Damage and Development 1 (REDD1) pathway offers another route to stop mTORC1. Metformin boosts REDD1 expression substantially in many cell types, including cancer cells [12]. REDD1 blocks mTORC1 by stabilizing the TSC1-TSC2-TBC1D7 inhibitory complex and freeing TSC2 from inhibitory 14-3-3 proteins.
Cells without p53 need REDD1 induction by metformin to block mTORC1. AMPK activation alone doesn’t work [12]. This shows how metformin’s different mechanisms work together to control cell growth.
Metformin also affects mTORC1 through Rag GTPases. These proteins sense amino acids and activate mTORC1 as heterodimers of RagA/B bound to RagC/D. Active GTPases normally bring mTORC1 to the lysosomal surface where Rheb activates it.
Scientists found that metformin blocks mTORC1 signaling by disrupting Rag GTPase function. This stops mTORC1 from reaching the lysosomal surface [13]. The discovery explains why metformin can block mTORC1 even in cells without AMPK, suggesting broader anti-aging benefits.
Impact on S6K and 4E-BP1 phosphorylation
Metformin’s blockade of mTORC1 changes protein synthesis through two key targets:
S6 Kinase (S6K): Metformin reduces S6K phosphorylation at Thr389. This limits its ability to activate ribosomal protein S6 [10]. The result is decreased synthesis of ribosomal proteins and translation machinery parts.
4E-BP1 (eIF4E-Binding Protein 1): Metformin dephosphorylates 4E-BP1, changing it from hyperphosphorylated (inactive) to hypophosphorylated (active) forms [14]. Active 4E-BP1 captures and holds eIF4E, blocking cap-dependent translation initiation.
These changes pack a punch. Liver cells treated with metformin through the TSC complex show a 75% drop in protein synthesis [11]. Even cells without TSC2 see a 25% reduction, showing how metformin uses multiple paths to control cell growth.
Polysome profiles back this up. They show proteins moving from polyribosomes to monoribosomes – a clear sign of translation suppression [14]. HT29 and HCT116 cancer cells showed dramatic drops in their polysome/monosome ratios after metformin treatment, from 1.85 to 0.64 and 0.35 to 0.17 [14].
These molecular mechanisms help explain why metformin mimics caloric restriction’s benefits – a proven way to extend lifespan. By reducing protein synthesis and energy use, metformin might create conditions that promote longevity, just like dietary restriction does.
Additional Resources:
- National Institute on Aging – TAME Trial Information
- Cell Metabolism Journal – mTOR Signaling Research
- Science Translational Medicine – Metformin and Aging Mechanisms
Metformin’s Role in Reducing Oxidative Stress and Inflammation
Metformin’s power to curb cellular aging comes from its reliable antioxidant and anti-inflammatory properties. This explains why researchers now see this diabetes medication as a promising longevity drug.
SKN-1/Nrf2 pathway activation
The SKN-1/Nrf2 pathway shows one of metformin’s most interesting antiaging mechanisms. Metformin activates SKN-1 (the worm equivalent of human Nrf2) in C. elegans worms. This transcription factor coordinates cellular defense against oxidative damage. The activation happens downstream of AMPK and needs the LKB1 tumor suppressor.
SKN-1/Nrf2 moves to the nucleus after activation. It binds to antioxidant response elements (AREs) in the promoter regions of many detoxification genes. This binding starts the expression of:
- Glutathione S-transferases
- NAD(P)H:quinone oxidoreductases
- Heme oxygenase-1
- Glutamate-cysteine ligase
This cascade helps metformin improve the production of endogenous antioxidants. The cells get better molecular “shields” against oxidative damage instead of just neutralizing free radicals directly.
Genetic studies show something remarkable. SKN-1 deletion completely stops metformin’s lifespan-extending effects in C. elegans. This proves how crucial this pathway is to metformin’s longevity benefits.
Reduction in ROS via mitochondrial complex I inhibition
Scientists first thought mitochondrial complex I inhibition was just a side effect. Now it seems central to metformin’s antiaging potential. Metformin creates a moderate reduction in reactive oxygen species (ROS) production by partially and reversibly inhibiting complex I (NADH:ubiquinone oxidoreductase).
This inhibition works through a balanced mechanism:
- Mild suppression of electron transport chain activity
- Decreased proton pumping across the inner mitochondrial membrane
- Reduced electron leakage during oxidative phosphorylation
- Lower superoxide generation at complexes I and III
The body responds well to this mild stress. It adapts and becomes more resilient. Research shows this mild mitochondrial stress activates quality control mechanisms. These include mitophagy (removal of damaged mitochondria) and better antioxidant defenses.
Metformin reduces ROS without affecting essential ATP production. Instead, it changes cellular metabolism to be more efficient. This mirrors what happens during caloric restriction—the most proven way to extend lifespan across species.
Anti-inflammatory gene expression modulation
Age-related diseases often stem from chronic low-grade inflammation, also known as “inflammaging.” Metformin fights this process by changing gene expression patterns that reduce inflammatory signaling.
Metformin blocks nuclear factor kappa B (NF-κB) at the molecular level. This master regulator of inflammatory responses gets inhibited through AMPK activation and other independent mechanisms:
- Less production of advanced glycation end products (AGEs)
- Blocked reactive nitrogen species generation
- Reduced inflammasome activation
- Lower levels of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α
These effects matter beyond diabetes treatment. Regular metformin use is associated with fewer inflammation markers in multiple tissues. This might explain why it helps with conditions from atherosclerosis to neurodegenerative diseases.
People who want to take metformin to live longer should note these anti-inflammatory effects. Moderate doses taken consistently over time might work best. This approach allows sustained reduction of inflammatory processes rather than short-term suppression.
These mechanisms show why metformin does more than just control blood sugar. It activates antioxidant defenses, reduces ROS production, and suppresses inflammatory pathways. This triple action targets multiple aspects of aging at their molecular roots.
Additional Resources:
- American Federation for Aging Research – TAME Trial Information
- National Institute on Aging – Metformin Research
- Cell Metabolism Journal – Oxidative Stress and Aging
Evidence from Invertebrate Models: C. elegans and Drosophila
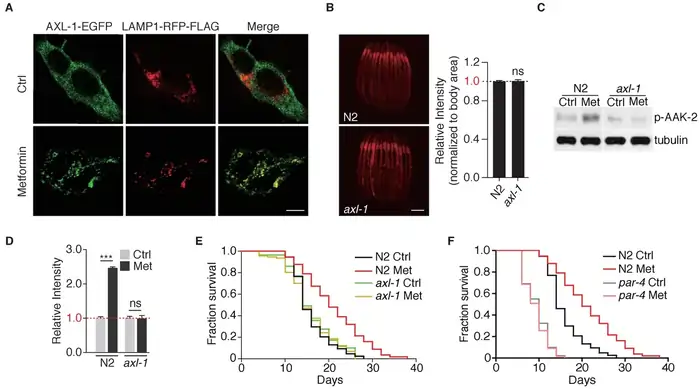
Image Source: eLife
Invertebrate models are vital testing grounds that show metformin’s longevity effects. These models reveal striking differences between species and explain the complexity of aging pathways.
Lifespan extension via LKB1–AMPK–SKN-1 in C. elegans
The tiny roundworm C. elegans shows impressive results with metformin. It extends their lifespan by 30-50%, making it one of the most effective treatments for this well-studied organism [15]. The lifespan extension works through a specific molecular pathway that needs three key players: LKB1 (the worm PAR-4), AMPK (AAK-2), and the transcription factor SKN-1 (the worm equivalent of mammalian Nrf2) [16].
Metformin activates AMPK through LKB1 in worms. This triggers SKN-1 to move into the nucleus where it activates detoxification genes [17]. The sort of thing I love is how metformin boosts expression of glutathione S-transferase 4 (gst-4) through AMPK—a vital step in the worm’s defense against oxidative stress [17].
The results are clear: removing aak-2 (AMPK) or skn-1 stops metformin’s lifespan-extending effects completely. This shows these proteins are essential [16]. Worms without these genes become much more sensitive to metformin’s toxic effects [17].
Microbiome-mediated effects on longevity
Scientists found something unexpected in worm studies. Metformin extends lifespan mostly by affecting gut bacteria rather than worm cells directly. It disrupts microbial folate and methionine metabolism in E. coli (the worms’ food source), which creates methionine restriction in the worm [17].
The worm’s AMPK pathway must be present to turn this altered bacterial metabolism into longer life. Scientists pre-treated E. coli with metformin before feeding them to worms. Normal worms lived 48% longer, while aak-2 mutants only gained 29% more lifespan [17]. This shows a fascinating interaction between drug, microbiome, and host metabolic pathways.
Lack of lifespan extension in Drosophila and possible toxicity
Fruit flies (Drosophila melanogaster) tell a different story. Multiple studies show they don’t live longer with metformin [18]. Low doses of metformin activate AMPK and reduce lipid stores in flies—just like in worms—but don’t increase their lifespan for either males or females [18].
Higher doses of metformin actually harm flies. At 100mM, male flies’ lifespan drops by 31% and females’ by 66% [18]. The biggest problem seems to be disrupted intestinal fluid balance. Metformin-treated flies produce concentrated fecal deposits that point to gut problems [19].
One study disagrees and reports longer lifespans from metformin in Drosophila, but mainly in age-related muscle deterioration [20]. This difference shows how metformin’s effects can vary greatly between tissues and experimental conditions.
Additional Resources:
- American Federation for Aging Research – TAME Trial
- National Institute on Aging – Metformin Research
- Buck Institute for Research on Aging – Invertebrate Models
Rodent Studies: Gender, Dose, and Age-Dependent Effects
Image Source: Aging-US
Studies on rodents show clear differences between genders and strains in their response to metformin. These findings give us a better picture of how it might work as an anti-aging treatment. Mice share many metabolic features with humans, unlike simpler organisms. This makes their responses much more relevant for understanding how metformin affects longevity.
HER-2/neu and SHR mouse models
Female HER-2/neu mice treated with metformin showed amazing benefits. The drug slowed down aging and tumor growth effectively [21]. Female spontaneously hypertensive (SHR) mice had impressive results too. Their mean lifespan increased by 37.8% with metformin (100 mg/kg in drinking water). The last 10% of survivors lived 20.8% longer, and maximum lifespan went up by 10.3% [22].
The results had some surprises. Metformin didn’t change estradiol levels or stop spontaneous tumors in female SHR mice [22]. HER-2/neu mice showed different results – their mean lifespan increased by 8% with metformin (0.5 mg/ml in drinking water) [23].
Timing of treatment onset and lifespan outcomes
The timing of metformin treatment plays a crucial role. Middle-aged male C57BL/6 mice lived 5.83% longer with 0.1% (w/w) metformin [24]. B6C3F1 male mice got the same dose and lived 4.15% longer [24]. Higher doses proved dangerous – 1% w/w shortened life by 14.4% because of kidney failure [24].
Young mice showed different results. Mice treated between 15-56 days old developed differently. Some changes were specific to their sex, while others weren’t. These early changes could affect how they age later in life [25].
Tumor suppression and metabolic improvements
Tumor development responses to metformin varied by strain. Female 129/Sv mice had 3.5 times fewer malignant tumors than untreated mice [26]. C57BL/6 mice on metformin showed better metabolic health. They had lower glycated hemoglobin, insulin, total cholesterol, and low-density lipoproteins [24].
Middle-aged male mice taking 0.1% metformin got stronger. They performed better in rotarod, treadmill, and open-field tests [24]. Their bodies became better at using fat for energy. This showed up as lower respiratory exchange ratios during day and night [24].
Additional Resources:
- National Institute on Aging – Interventions Testing Program
- Buck Institute for Research on Aging – Metformin Studies
- American Federation for Aging Research – TAME Trial
Human Observational Studies and the TAME Trial

Image Source: NutritionFacts.org
“Indeed, compared to diabetics who take drugs other than metformin, those on metformin appear protected from age-related susceptibilities like fractures or serious infection, glaucoma, and cognitive impairment.”
— Michael Greger, Physician, author, and founder of NutritionFacts.org
The jump from animal studies to human trials of metformin as an anti-aging treatment shows both exciting possibilities and major challenges. Scientists continue their randomized clinical trials focused on aging, while existing data hints at some fascinating potential.
Lower mortality in diabetic patients on metformin
Research looks promising. A detailed review of 53 studies showed that people who take metformin live longer, with fewer deaths from age-related conditions like cancer and heart disease [27]. The numbers tell an interesting story – diabetics who took metformin lived longer than people without diabetes (hazard ratio=0.93, 95%CI 0.88-0.99) [28]. The results become even more striking when you compare metformin users to diabetics using other treatments: non-metformin therapies (HR=0.72, 95%CI 0.65-0.80), insulin (HR=0.68, 95%CI 0.63-0.75), or sulphonylurea (HR=0.80, 95%CI 0.66-0.97) [28].
A 2014 study revealed that Type 2 diabetes patients on metformin had better survival rates than non-diabetics [29]. However, a 2022 analysis painted a different picture. This study showed that over twenty years, Type 2 diabetes patients who took metformin ended up with shorter survival times than similar non-diabetic people [30].
Design and goals of the TAME trial
The Targeting Aging with Metformin (TAME) Trial breaks new ground in aging research:
- Scientists designed a six-year, double-blind, placebo-controlled multicenter trial with 3,000 participants aged 65-79 [31]
- Each participant takes 1500 mg of metformin daily, with average follow-up lasting more than 3.5 years [32]
- Researchers track how long it takes for age-related health issues to appear (heart disease, cancer, cognitive decline, and death) [32]
TAME stands out from typical single-disease studies. It wants to prove that medical treatment can target aging itself [31]. Success could lead the FDA to recognize aging as something doctors can treat – this would mean a fundamental change in how medicine approaches age-related conditions [31].
Challenges in proving anti-aging effects in humans
Money remains the biggest hurdle for TAME right now. The NIH has put in about $5 million, but the study needs $45-70 million to finish [33]. Since metformin has no patent protection, drug companies see little reason to fund this research [33].
Dosing creates another challenge. Scientists used metformin doses 10-100 times higher than what humans can safely have in their blood during early studies [32]. This raises questions about whether safe doses can actually slow down aging.
If you’re thinking about taking metformin to live longer, remember these unanswered questions. It’s best to wait for clinical trial results before deciding whether healthy adults should take this medication.
Additional Resources:
- American Federation for Aging Research: afar.org/tame-trial
- National Institute on Aging: nia.nih.gov
- Cell Metabolism Journal: cell.com/cell-metabolism
Safety Profile, Dosage, and Considerations for Longevity Use
You need to understand metformin’s safety profile if you want to benefit from its longevity effects. People’s growing interest in metformin and longevity makes it vital to know its common side effects and rare risks.
Common side effects: GI distress, lactic acidosis risk
Metformin most often causes gastrointestinal discomfort, which affects about 20-30% of patients [34]. People might experience nausea, bloating, diarrhea, stomach pain, and sometimes vomiting [35]. The good news is these effects usually get better over time. Taking metformin with meals or starting with lower doses can help reduce these effects [36].
Some people who use metformin for a long time might develop vitamin B12 deficiency, which affects 6-30% of users [27]. This type of B12 deficiency is usually milder than other forms and rarely leads to neuropathy or anemia [32].
Lactic acidosis is very rare, with only 6 cases per 100,000 patient-years [37]. Yet it remains metformin’s most dangerous possible complication. Half of the people who develop this condition don’t survive [32]. Your risk increases if you have kidney problems, liver disease, alcoholism, or conditions that reduce oxygen levels [38].
Dosing strategies in animal vs. human studies
The difference in doses between lab studies and human use matters a lot in metformin aging research. Lab studies often use metformin levels 10-100 times higher than what humans can achieve in their blood [32]. People without diabetes who take a 1g dose reach blood levels of about 25μM within 3 hours. Diabetic patients taking 1g twice daily reach peak levels around 40μM [39].
Mouse studies showed interesting results. A 0.1% (w/w) metformin dose helped mice live 5.83% longer. However, a higher dose (1% w/w) turned out to be harmful and shortened their lives by 14.4% because of kidney problems [40].
Considerations for off-label use in healthy individuals
If you want to take metformin for longevity without having diabetes, you should take some precautions. Doctors usually start with 500mg daily to check how well you tolerate it. They might gradually increase the dose up to 2000mg daily [34]. You need regular checks of your kidney function, vitamin B12 levels, and lactic acid when using metformin off-label [34].
Metformin might not work the same way for everyone because of its complex effects on the body. Many experts suggest a customized approach that looks at your existing health conditions, other medications, and keeps track of specific health markers [34].
Additional Resources:
- American Federation for Aging Research – TAME Trial
- National Institute on Aging – Metformin Research
- FDA Prescribing Information for Metformin
Conclusion
Metformin sits right at the sweet spot between diabetes treatment and possible life extension. This piece has delved into compelling evidence from many sources—from amazing lifespan extension in C. elegans to more subtle results in rodents and human observations. Scientists’ growing interest in metformin’s antiaging properties stems from its power to activate AMPK, inhibit mTORC1, cut down oxidative stress, and control inflammation.
In spite of that, big questions still need answers. The way metformin affects different species tells an intriguing story—it’s a big deal as it means that worms live much longer, while higher doses might harm Drosophila. This shows how complex aging can be across organisms. On top of that, rodent studies reveal that gender, dose, and timing all matter, which suggests we need individual-specific approaches instead of one-size-fits-all prescriptions.
The TAME trial marks a defining moment in aging research. The results could change medicine forever if they show metformin can delay multiple age-related diseases at once. This would spark a fundamental change from treating diseases one by one to targeting why aging happens in the first place.
Anyone excited about metformin’s potential needs to stay patient. You should talk to healthcare providers who know your medical history before thinking over metformin for longevity. They can weigh the risks and benefits for your specific case. The best dose for fighting aging might not match what works for diabetes—we’ll know more once clinical trials wrap up.
Research might show that metformin works best with other approaches like exercise or diet changes. The evidence looks good so far, especially since people taking it tend to live longer. But we need solid clinical trials to prove metformin really fights aging in healthy humans.
Resource Links:
- American Federation for Aging Research – TAME Trial
- National Institute on Aging – Metformin Research
- FDA Prescribing Information for Metformin
FAQs
How does metformin potentially extend lifespan?
Metformin may extend lifespan by activating AMPK, inhibiting mTORC1, reducing oxidative stress, and modulating inflammation. These mechanisms mimic some effects of caloric restriction, a well-established intervention for extending lifespan across species.
Is metformin safe for long-term use in healthy individuals?
While metformin is generally safe for long-term use in diabetics, its safety profile for healthy individuals seeking longevity benefits is still being studied. Common side effects include gastrointestinal discomfort, and there’s a rare risk of lactic acidosis. Consult a healthcare provider before considering off-label use.
What is the TAME trial and why is it significant?
The Targeting Aging with Metformin (TAME) trial is a groundbreaking study aiming to prove that aging itself can be treated medically. It’s significant because if successful, it could lead to FDA recognition of aging as an approved treatment indication, potentially revolutionizing approaches to age-related diseases.
How does metformin’s effect on lifespan vary between species?
Metformin’s effects on lifespan vary dramatically between species. It extends lifespan by 30-50% in C. elegans worms but shows no benefit in fruit flies. In mice, results are mixed and depend on factors like gender, dose, and timing of treatment.
What is the recommended dosage of metformin for potential longevity benefits?
The optimal dosage of metformin for longevity benefits in humans is not yet established. Clinical studies typically use doses ranging from 500mg to 2000mg daily. However, the ideal dosage may differ from diabetes treatment protocols and should be determined under medical supervision based on individual factors.
References
[1] – https://www.nature.com/articles/s41586-022-04431-8
[2] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5805978/
[3] – https://www.nature.com/articles/ncomms5535
[4] – https://www.sciencedirect.com/science/article/abs/pii/S1096719207004003
[5] – https://www.sciencedirect.com/science/article/pii/S000291652302169X
[6] – https://link.springer.com/article/10.1007/s00125-017-4342-z
[7] – https://onlinelibrary.wiley.com/doi/full/10.1002/cbin.10915
[8] – https://www.nature.com/articles/emm201681
[9] – https://www.sciencedirect.com/science/article/pii/S1550413116306428
[10] – https://aacrjournals.org/cancerres/article/67/22/10804/533582/Metformin-Inhibits-Mammalian-Target-of-Rapamycin
[11] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5299044/
[12] – https://aacrjournals.org/cancerres/article/71/13/4366/568093/Metformin-Independent-of-AMPK-Induces-mTOR
[13] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3081779/
[14] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6210051/
[15] – https://elifesciences.org/articles/31268
[16] – https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0008758
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3898468/
[18] – https://pubmed.ncbi.nlm.nih.gov/23077661/
[19] – https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0047699
[20] – https://pubmed.ncbi.nlm.nih.gov/36394755/
[21] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4497590/
[22] – https://pubmed.ncbi.nlm.nih.gov/18728386/
[23] – https://www.tandfonline.com/doi/pdf/10.4161/cc.9.1.10407
[24] – https://www.nature.com/articles/ncomms3192
[25] – https://www.sciencedirect.com/science/article/abs/pii/S004763742100169X
[26] – https://www.aging-us.com/article/100245/text
[27] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8374068/
[28] – https://pubmed.ncbi.nlm.nih.gov/28802803/
[29] – https://www.nytimes.com/2025/05/01/well/metformin-aging-longevity-benefits-risks.html
[30] – https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-023-15764-y
[31] – https://www.afar.org/tame-trial
[32] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6779524/
[33] – https://www.npr.org/sections/health-shots/2024/04/22/1245872510/a-cheap-drug-may-slow-down-aging-a-study-will-determine-if-it-works
[34] – https://montecitoconciergemedicine.com/is-it-safe-to-take-metformin-to-fight-aging/
[35] – https://www.nhs.uk/medicines/metformin/side-effects-of-metformin/
[36] – https://www.webmd.com/diabetes/metformin-side-effects
[37] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4264704/
[38] – https://www.medsafe.govt.nz/profs/puarticles/5.htm
[39] – https://academic.oup.com/edrv/article/42/1/77/5902802
[40] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3736576/

![NMN vs NR: Which Anti-Aging Supplement Actually Works? [2025 Science] 13 NMN vs NR](https://longevityblueprinthealth.com/wp-content/uploads/2025/05/810384d5-0f16-477a-abe0-0872e930cf72-1.webp)